Clemmensen Reduction of Ketones in Anhydrous Organic Solvents
Abstract
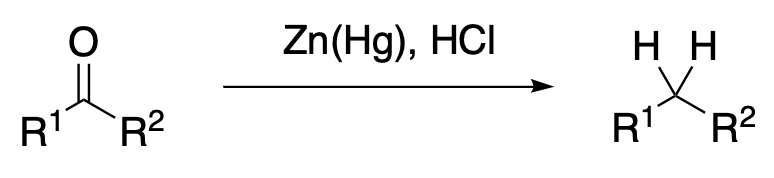 Graphical Abstract by Sara Hunihan
Graphical Abstract by Sara Hunihan
The Clemmensen reduction of ketones and aldehydes using zinc and hydrochloric acid is the simplest direct method for converting the carbonyl group into a methylene group. Typically the carbonyl is refluxed for several hours with 40% hydrochloric acid, amalgamated zinc, and a water-immiscible organic solvent such as toluene. Because of these harsh conditions, reports of successful Clemmensen reduction of polyfunctional ketones have been rare. A milder procedure using dry hydrogen chloride in organic solvent extends the potential of this reaction. Other developments that define scope of both aqueous and anhydrous reduction conditions are discussed and an effort is made to compare the properties of possible reduction intermediates with other organozinc species.

