Desulfurization with Raney Nickel
Abstract
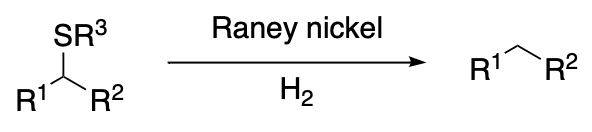 Graphical Abstract by Nathan Mitchell
Graphical Abstract by Nathan Mitchell
The first example of the desulfurization of an organic compound by means of Raney nickel was reported by Bougault in 1940. Since that time the reaction has been used with much success both for synthesis and the determination of structure. In general, a Raney nickel desulfurization involves the breaking of a carbon–sulfur bond in an organic substance and, usually, the formation of at least one new carbon–hydrogen bond. The oxidation state of the sulfur that is removed may vary from two to six. Although the hydrogenolysis of organic compounds containing sulfur has been accomplished by a variety of inorganic reagents, this survey has been restricted to desulfurizations brought about by Raney nickel in which adsorbed hydrogen usually, but not always, has been retained. The symbol “Ni(H)” will be used to indicate such a reagent. Desulfurizations effected by nickel-aluminum alloy and aqueous alkali (Schwenk-Papa reduction) are also included.

