Hydrocyanation of Conjugated Carbonyl Compounds
Abstract
Graphical Abstract by Alivia Glas
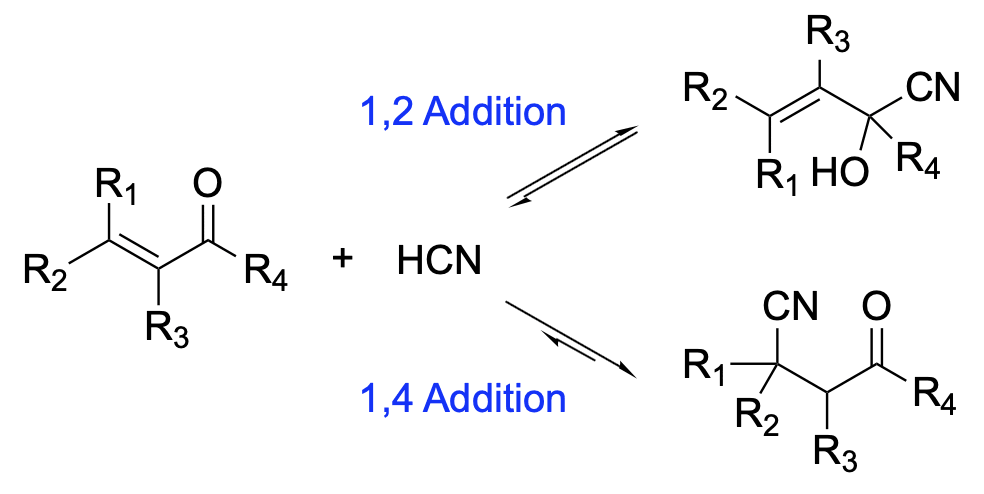
Hydrogen cyanide adds to a multiple bond in the presence of an appropriate catalyst. The process is called hydrocyanation. The addition to an α,β-unsaturated carbonyl compound gives a 1,2 adduct, α-cyanohydrin, and a 1,4 adduct, β-cyano ketone. Both 1,2 and 1,4 additions are reversible in principle, but in the presence of a base the reverse 1,2 addition is much faster than the reverse 1,4 reaction, leading to the 1,4 adduct as a major (usually sole) product.
In this sense conjugate hydrocyanation is a special case of the Michael reaction. Predominance of 1,2 or 1,4 addition depends on the substrate structure, the reagent, and the reaction conditions. In this chapter 1,2 addition is discussed only when necessary. This chapter deals chiefly with the 1,4 addition and, more generally, with conjugate addition including that on conjugated polyethylenic compounds giving 1,6 or more extended conjugated adducts. Carbonyl substrates include ketones, aldehydes, carboxylic acid derivatives, carbonitriles, and carboimines. Also included are β-functionalized carbonyl compounds, and π-allylpalladium complexes, that generate α,β-unsaturated carbonyl compounds, under hydrocyanation conditions. Although hydrogen cyanide adds to α,β-unsaturated compounds activated by other groups such as nitro and sulfone, these reactions are not considered in this chapter.
The first example of 1,4 addition of hydrogen cyanide to α,β-unsaturated carbonyl derivatives appeared more than a century ago, in 1873, when Claus prepared tricarballylic acid (1,2,3-propanetricarboxylic acid) by heating an ethanolic solution of 2,3-dichloropropene and potassium cyanide in a sealed tube.
The discovery in 1903 that the reactive species in hydrocyanation is the cyanide anion, CN−, and that the rate of the reaction is proportional to the concentration of CN−8, formed the foundation for active study of hydrocyanation chemistry. A fundamental procedure for conjugate hydrocyanation was developed which used 2 molar equivalents of potassium cyanide and 1 molar equivalent of acetic acid in aqueous alcohol. The reaction mode was clarified by Ingold and Michael in the 1930s. Many chemists began extensive application of the reaction to organic syntheses, and attempts were made to explore improved procedures with higher efficiency and fewer side reactions. Detailed reviews cover developments of conjugate hydrocyanations up to the 1950s.
In recent years conjugate hydrocyanation has been used in total syntheses of complex natural products such as terpenes, steroids, and alkaloids. However, conventional procedures using hydrogen cyanide in the presence of a base or an alkali or alkaline-earth metal cyanide were not efficient. Furthermore, the efficiency could not be improved without increasing side reactions. The difficulty was overcome in 1962 when methods using organoaluminum compounds were discovered by Nagata and co-workers. They developed two new hydrocyanation methods, one involving a combination of hydrogen cyanide and an alkylaluminum and the other an alkylaluminum cyanide, both in an aprotic solvent. Applications of the new methods have led to total syntheses of complex natural products having a methyl, a functionalized methyl, or a carbon bridge at an angular position.

