Oxidative Cleavage of Furans
Abstract
 Graphical Abstract by Carrie Twente
Graphical Abstract by Carrie Twente
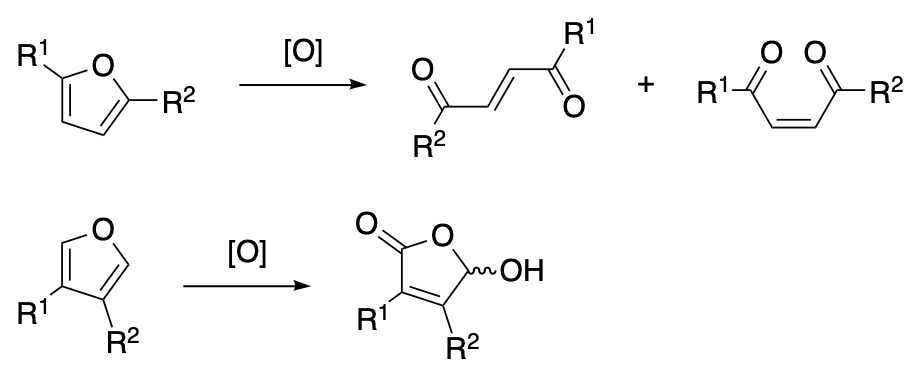
Oxidative ring cleavage reactions of furans constitute a group of important transformations that have found wide utility in synthetic organic chemistry. These processes have been used to prepare a variety of 1,4-dicarbonyl compounds including 1,4-dioxoalkenes, 4-oxoalkenals, 4-oxoalkenoic acids, and their derivatives such as 4-hydroxybutenolides and 2,5-dialkoxydihydrofurans. Oxidations of furfuryl alcohols (Achmatowicz reaction) and furfuryl amines (aza-Achmatowicz reaction) provide access to highly functionalized heterocyclic structures that have been employed as intermediates in synthetic routes for the preparation of complex molecules including carbohydrates and alkaloids. Complete oxidative degradation of the furan ring affords carboxylic acids; thus the oxygen heterocycle has served as a masked carboxyl group in many synthetic studies. These transformations and their applications in total syntheses are covered in this chapter.

