The Michael Reaction
Abstract
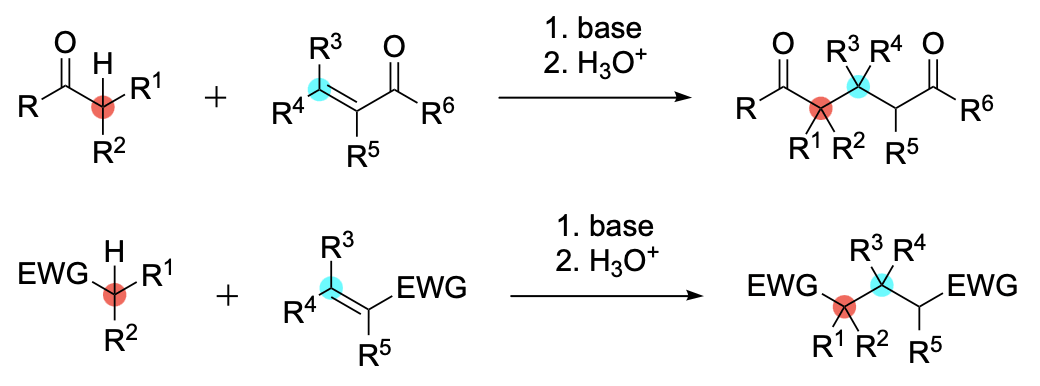 Graphical Abstract by Alexandra Seesee
Graphical Abstract by Alexandra Seesee
The Michael condensation in its original scope is the addition of an addend or donor containing an alpha-hydrogen atom in the system O=C–CH to a carbon-carbon double bond that forms part of a conjugated system of the general formula C=C–C=O in an acceptor. The condensation takes place under the influence of alkaline reagents, typically alkali metal alkoxides. The range of addends is very broad. Typical acceptors are alpha, beta-unsaturated aldehydes, ketones, and acid derivatives. As an extension of the original scope, the Michael condensation has come to be understood to include addends and acceptors activated by groups other than carbonyl and carboxalkoxy. The wider scope is encompassed in this survey.

