The Mitsunobu Reaction
Abstract
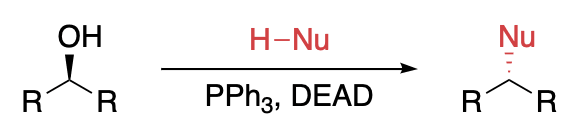 Graphical Abstract by Samantha Yu
Graphical Abstract by Samantha Yu
Alkyl and aryl phosphites and phosphines react with compounds having weak heteroatom–heteroatom bonds, such as S—S, O—O, etc., and with azo compounds to form reactive phosphonium salts. These phosphonium salts in turn promote “redox” condensation reactions with compounds having active hydrogens. The condensation reaction of alcohols using the redox couple of a triaryl- or trialkylphosphine and a dialkyl azodicarboxylate has become known as the Mitsunobu reaction, based on his pioneering work in the late 1960s. The overall reaction is summarized, wherein the alcohol (R1OH) and acidic compound (H–Nu) are condensed to form product (R1–Nu), while triphenylphosphine is oxidized to triphenylphosphine oxide and the azodicarboxylate is reduced to the hydrazine. Although the typical redox combination is diethyl azodicarboxylate (DEAD) and triphenylphosphine, many other combinations have found selected use. The reaction is generally limited to primary and secondary alcohols, although tertiary alcohols react in a few intramolecular and intermolecular reactions. For secondary alcohols the reaction usually proceeds with clean inversion of stereochemistry. The acidic component of the reaction generally has an aqueous pKa < 15, with intramolecular reactions providing the exceptions. Examples of H–Nu include oxygen nucleophiles such as carboxylic acids and phenols; nitrogen nucleophiles such as imides, hydroxamates, and heterocycles; sulfur nucleophiles such as thiols and thioamides; and carbon nucleophiles such as β-ketoesters.
Major reviews of the Mitsunobu reaction were published in 1981 by Mitsunobu and in 1983 by Castro. The former review concentrated on reactions using DEAD/triphenylphosphine, while the latter review focused on reactions in which halogens replaced the hydroxy group using reagents such as triphenylphosphine/carbon tetrachloride, triphenyl phosphite/iodomethane, and triphenylphosphine/N-halosuccinimide. Reactions involving the DEAD/triphenylphosphine redox system are the principal subject of this chapter, with emphasis on the literature between 1981 and 1988.

