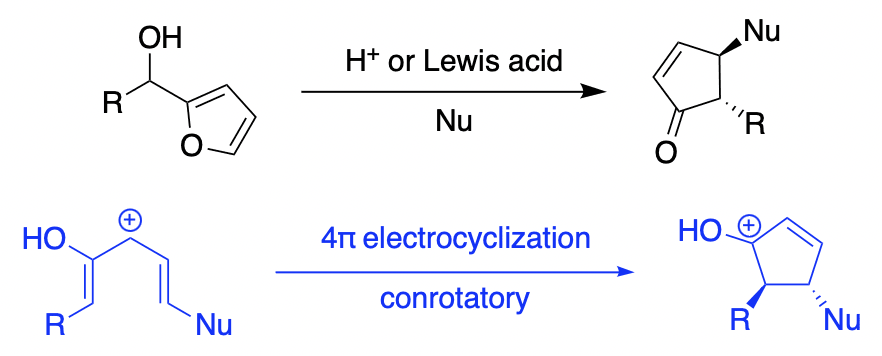
The Piancatelli Reaction
Abstract
 Graphical Abstract by Morgan Lockett
Graphical Abstract by Morgan Lockett
The Piancatelli reaction allows the synthesis of 4-substituted cyclopentenone derivatives from furylcarbinols and various nucleophiles such as water, alcohols, or amines. Easily accessible classes of products produced by this methodology include 4-hydroxy-, 4-alkoxy- and 4-aminocyclopent-2-enones. Cascade Piancatelli reactions involving intramolecular conjugate additions to the resulting enone moiety can lead to polycyclic products. Since the development of catalytic versions of the reaction using Lewis and Brønsted acids, the methodology has attracted considerable attention and has found several applications in the synthesis of complex molecules, including natural products. Enantioselective Piancatelli reactions are also possible by utilizing chiral Brønsted acids as catalysts. This chapter describes in detail the proposed mechanisms as well as the scope and limitations of the Piancatelli reaction.

