Radical Allylation, Vinylation, Allenylation, Alkynylation, and Propargylation Reactions Using Tin Reagents
Abstract
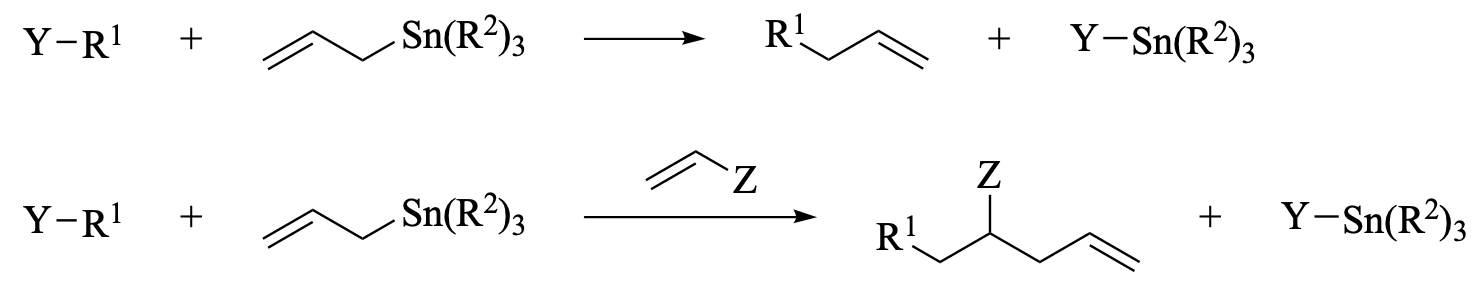
This chapter describes the use of allyl- and vinylstannane reagents for a variety of radical-based transformations. The processes discussed include direct addition of radicals to allyl- and vinylstannanes, multicomponent or multistep cascade reactions that terminate with an intermolecular allylation or vinylation, cyclizations onto allyl- or vinylstannane moieties, and allylstannylation reactions. Allylation reactions have provided a framework for the exploration of stereoselective radical reactions, and models for explaining the stereoselectivity obtained by a number of approaches are presented. Examples are provided to illustrate the scope of applications of allyl- and vinylstannane radical reactions in synthesis, as well as the ways that the newly introduced allyl and vinyl groups have been subsequently manipulated. A comparison is made to radical allylation and vinylation reactions that employ non-tin-based reagents.

