The [2,3]-Wittig Rearrangement
Abstract
 Graphical Abstract by Ariya Desai
Graphical Abstract by Ariya Desai
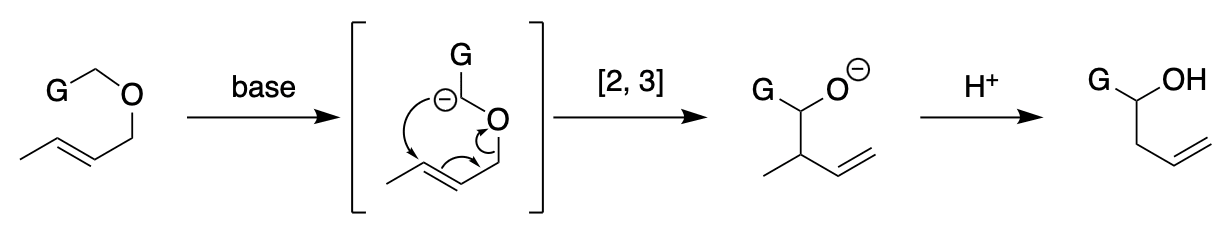
The [2,3]-sigmatropic rearrangement, constitutes a versatile type of bond reorganization which encompasses a number of variations in terms of an atom pair (X, Y) and the type of electron pair on Y (anions, nonbonding electron pairs, or ylides). The Sommelet–Hauser rearrangement is representative.
This chapter focuses on the special class of [2,3]-sigmatropic rearrangement that involves an oxycarbanion (X = oxygen, Y = carbanion) as the migrating terminus. This type of rearrangement is now termed the [2,3]-Wittig (sigmatropic) rearrangement. The reaction name clearly originates from the fact that this rearrangement formally represents a [2,3]-sigmatropic version of the classic Wittig rearrangement, a well-known 1,2-alkyl shift of oxycarbanions. The [2,3]-Wittig rearrangement has a rather recent history. Perhaps the first observation of the [2,3]-Wittig shift is the rearrangement of the allyl fluorenyl ether, which was made in 1960 in the context of mechanistic studies on the Wittig rearrangement. The period of the 1960s to the early 1970s witnessed slow progress with a focus on mechanistic studies mainly of allyl benzyl ether systems. The synthetic power of this carbanion rearrangement as a general method was recognized when Still (1978) and Nakai (1981) established the highly stereoselective variants of the genuine [2,3]-Wittig rearrangement. In recent years the [2,3]-Wittig rearrangement has enjoyed widespread application in many facets of organic synthesis. Various aspects of the reaction have been reviewed.
This chapter deals with the mechanism, scope and limitation, stereochemistry, and synthetic applications of the [2,3]-Wittig rearrangement with emphasis on the stereochemical aspects and the synthetic utility. Other hetero [2,3]-Wittig rearrangements such as thio-[2,3]-Wittig variants are not covered.

