Enantioselective Pictet-Spengler Reactions
Abstract
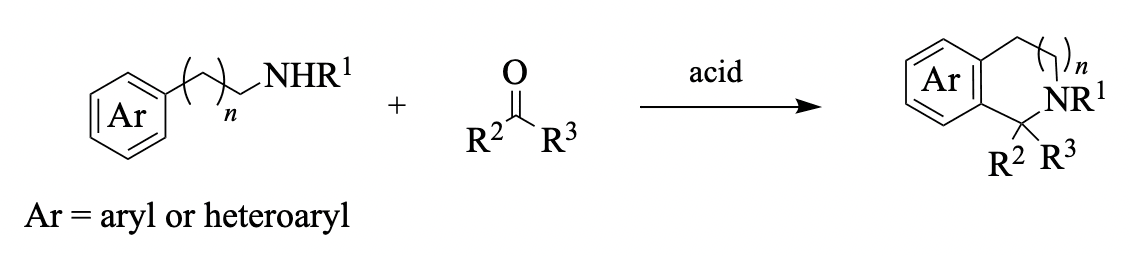
Enantioselective Pictet–Spengler reactions are promoted or catalyzed by small chiral molecules, including Brønsted acids, Lewis acids, and hydrogen-bond donors (e.g., thiourea compounds). While tryptamines and β-phenethylamines are commonly used in these reactions, a variety of other aryl-group-containing amines are also viable substrates. Achiral starting materials can participate in cascade reaction sequences that include an enantioselective Pictet–Spengler step. Mechanistic insights for these transformations are provided whenever possible. The Pictet–Spengler reaction allows access to heterocyclic products that are otherwise assembled via less direct methodologies. These products are employed as key intermediates in the synthesis of natural products such as yohimbine, arboricine, corynantheidine, mitragynine, harmicine, peganumine A, deplancheine, arborescidine C, and crispine A. The literature coverage for this Chapter extends to December 2019.

