Hydrozirconation of Alkynes
Abstract
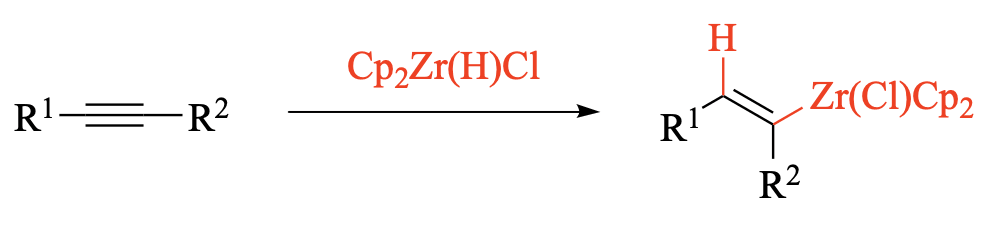
Since the first preparation of organozirconocenes from alkenes and alkynes in 1972, hydrozirconation has become one of the most commonly used stoichiometric methods to convert readily available starting materials into reactive but stable organometallic intermediates. A broad range of subsequent transformations may be employed to convert organozirconocenes in situ into high-value functionalized products, often with the strategic formation of one (or several) new carbon–carbon bonds.
This chapter focuses on the hydrozirconation of terminal and internal alkynes, and the subsequent synthetic transformations of the resulting alkenylzirconocenes. Zirconocene hydrochloride, Cp2Zr(H)Cl, is most frequently employed for the hydrozirconation step and can be used as a reagent or prepared in situ. Subsequent reactions of alkenylzirconocenes include additions to inorganic electrophiles such as halogens, as well as in situ ligand transfers to other metals, such as palladium and zinc, that further expand the range of accessible bond formations. The initial discussion focuses on the mechanism and stereochemical considerations, and on the steric and electronic factors that determine the regiochemistry of the either kinetically or thermodynamically controlled hydrozirconation. The “Scope and Limitations” section presents information on functional group compatibilities and is organized by the type of synthetic transformation of alkenylzirconocenes. Representative applications of the hydrozirconation of alkynes are showcased in syntheses of natural products, comparisons to alternative methods for the hydrometallation of alkynes.
The goal of this chapter is to demonstrate the significance of hydrozirconation in organic synthesis, including the utility of the stoichiometric zirconium organometallics obtained from alkyne substrates, and to provide inspiration for the future development of new synthetic methods and strategies.

