The Arndt-Eistert Reaction
Author(s):
Bachmann, W. E.; Struve, W. S.
Volume:
1
Published:
1942
Abstract
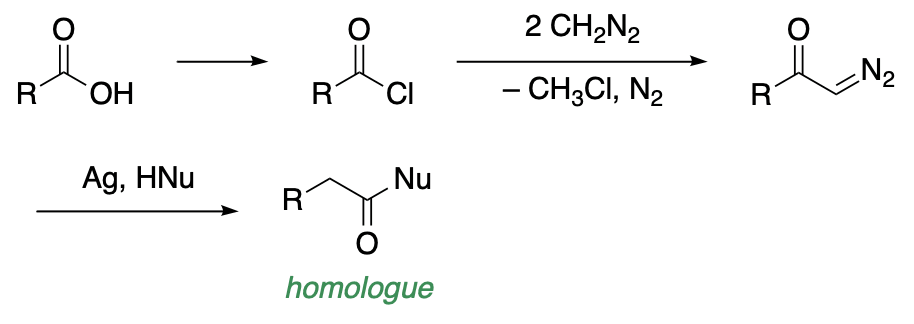 The Arndt-Eistert synthesis is a procedure for converting an acid to its next highest homolog or to a derivative of the homologous acid, and an ester and an amide. The synthesis, which is applicable to both, aliphatic and aromatic acids involves the following three operations; formation of the acid chloride; reaction of the acid chloride with diazomethane to yield a diazoketone; and rearrangement of the diazoketone in the presence of suitable reagents and a catalyst (colloidal silver, platinum, copper). An acid is formed in the presence of water, and ester is produced in an alcohol, and am amide results when ammonia or an amine is used.
The Arndt-Eistert synthesis is a procedure for converting an acid to its next highest homolog or to a derivative of the homologous acid, and an ester and an amide. The synthesis, which is applicable to both, aliphatic and aromatic acids involves the following three operations; formation of the acid chloride; reaction of the acid chloride with diazomethane to yield a diazoketone; and rearrangement of the diazoketone in the presence of suitable reagents and a catalyst (colloidal silver, platinum, copper). An acid is formed in the presence of water, and ester is produced in an alcohol, and am amide results when ammonia or an amine is used.

