The Amination of Heterocyclic Bases by Alkali Amides
Abstract
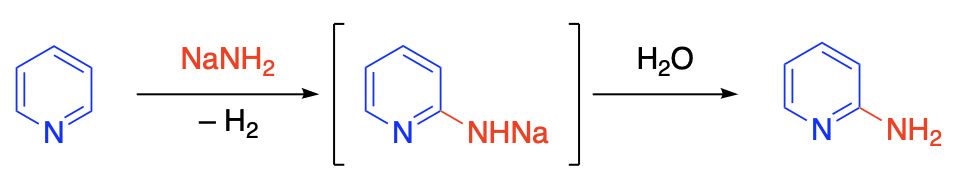
Heterocyclic bases such as pyridine and quinoline and their derivatives react with metal amides to yield amino derivatives. For example, pyridine is converted to 2-aminopyridine by the action of sodium amide; an intermediate metal derivative is formed, and this is hydrolyzed to the free amine. It has been suggested that the initial step in the reaction is the addition of the metal amide to the –CH=N– group; the resulting product is then transformed to the metal derivative of the amine, either through intramolecular rearrangement or through decomposition to the amino compound and sodium hydride to give the metal derivative.

