The Reformatsky Reaction
Abstract
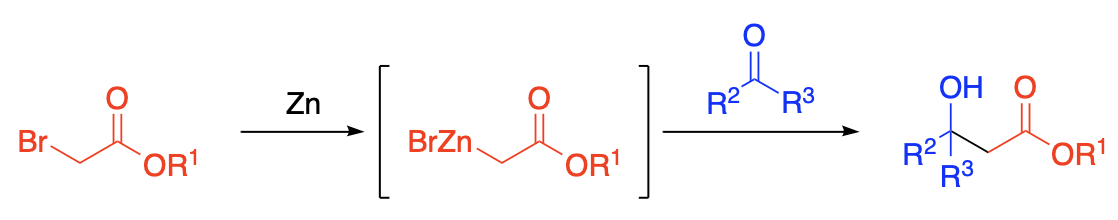
The reaction which takes place between a carbonyl compound such as an aldehyde, ketone, or an ester, and an alpha-haloester in the presence of zinc is commonly known as the Reformatsky reaction. It represents an extension of the reactions of carbonyl compounds with a dialkylzinc or an alkylzinc halide, but possesses the advantage that the isolation of the organozinc compound is unnecessary. The process creates a new carbon-carbon linkage and involves the following: Formation of an organozinc halide; addition to the carbonyl group of the aldehyde or ketone, decomposition by dilute acids.

