The Elbs Reaction
Abstract
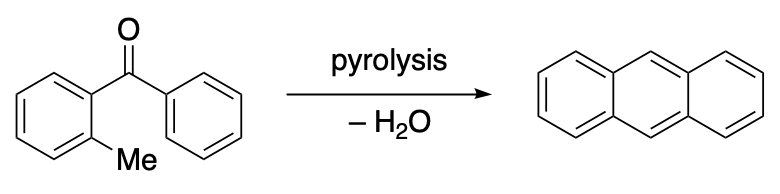
Diaryl ketones having a methyl or methylene substituent adjacent to the carbonyl group often suffer cyclodehydration when submitted to pyrolysis and afford a certain amount of the corresponding anthracene derivative. The reaction is usually carried out by heating the ketone without catalyst or solvent at the reflux temperature or at a temperature in the range of 400-450 degrees until the water is no longer evolved. The main hydrocarbon reaction product may not be that normally expected based on the starting material. A product of the Elbe condensation usually require extensive purification. The mechanism of the condensation is not known.

