The Fries Reaction
Abstract
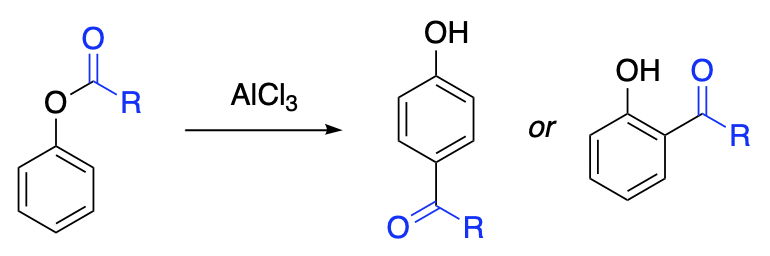
The Fries reaction consists in the conversion of an ester of a phenol to an ortho– or para-hydroxyketone, or a mixture of both, by treatment with aluminum chloride. The Fries reaction is considered to be a true intramolecular rearrangement in which the acyl group shifts directly from the oxygen atom to the carbon atom of the ring. Although the products of this reaction can also be prepared via Friedel-Crafts acylation of phenols, overall yields of a two-step sequence involving acylation followed by Fries reaction are generally higher than direct acylation of phenols for the preparation of phenolic ketones.

