Reductive Cyclization of 2-Nitro- and β-Nitrostyrenes, 2-Nitrobiphenyls, and 1-Nitro-1,3-Dienes to Indoles, Carbazoles, and Pyrroles
Author(s):
Berkowitz, W.; Söderberg, B. C. G.
Volume:
111
Pages:
417 – 769
Published:
2022
Abstract
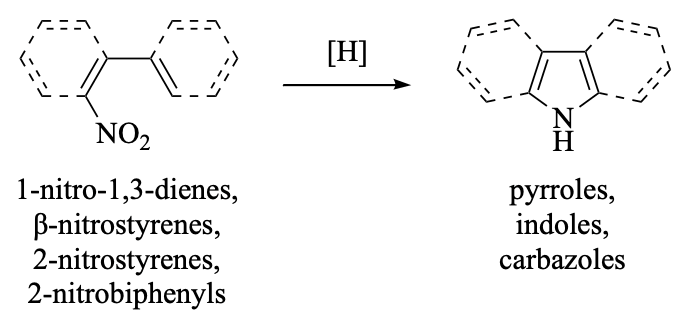
This chapter presents a comprehensive review of reductive cyclizations of 2-nitro- and β-nitrostyrenes, 2-nitrobiphenyls, and 1-nitro-1,3-dienes to furnish indoles, carbazoles, and pyrroles, respectively, as well as heteroatom analogs thereof. Two variations of the reductive cyclization are discussed: the Cadogan–Sundberg reaction, which is mediated by ternary phosphorus compounds, and the Watanabe–Cenini–Söderberg reaction, which employs a palladium catalyst in the presence of carbon monoxide. A few closely related reductive cyclizations are also presented, as are comparisons with other cyclizations that form the nitrogen–carbon bond of indoles and derivatives.

